KISQALI single-strength tablets make dose reduction simple and convenient
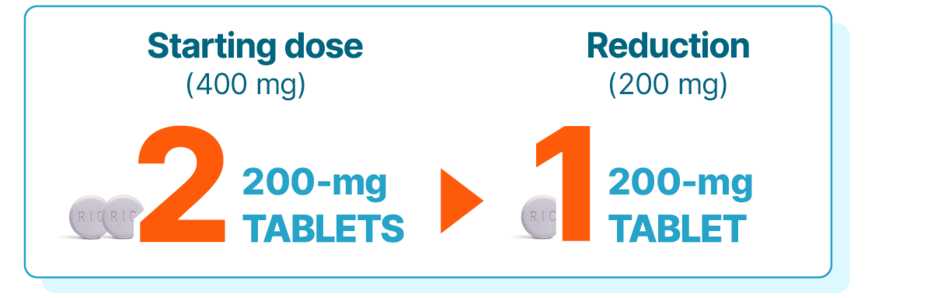
DOSE REDUCTIONS
Dose reductions with KISQALI mean no need for new mid-cycle prescriptions or additional costs
For patients with stage II/III HR+/HER2- eBC at high risk of recurrence,
KISQALI is given as 400 mg (2 x 200-mg tablets) orally, once daily (3 weeks on, 1 week off) for 36 months with an AI1
Review the full Prescribing Information for recommended dosing of selected AI
An LHRH agonist should be used concomitantly with AI in men and premenopausal women
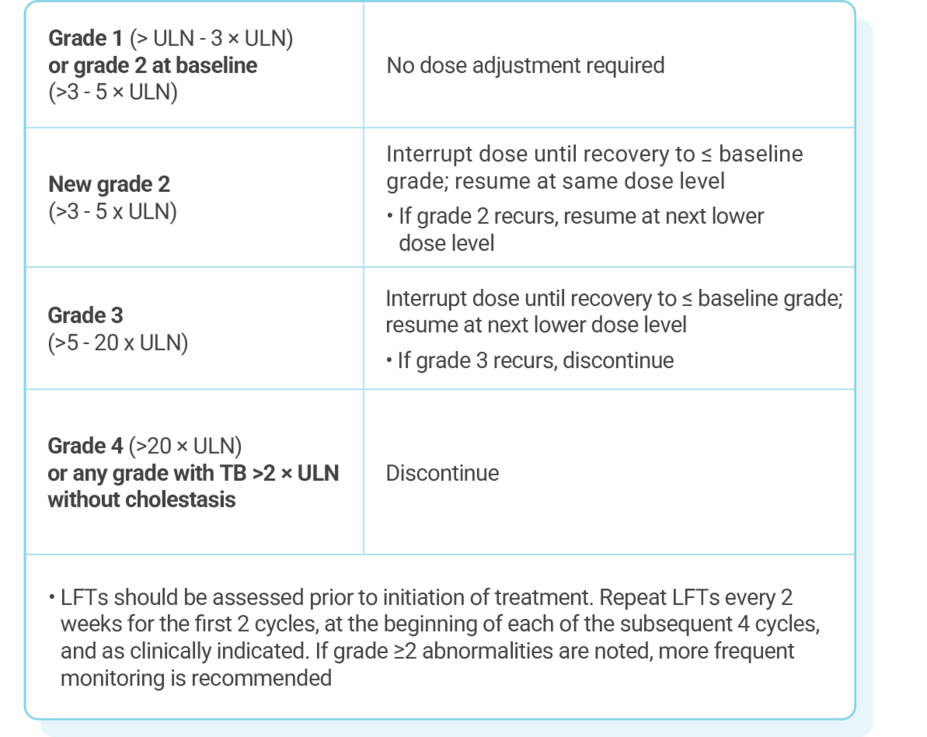
Dose adjustments for adverse reactions should be made by reducing the number of tablets taken1
If dose reduction below 200 mg/day is required, discontinue treatment
KISQALI dose modification is recommended based on individual safety and tolerability
KISQALI can be taken with or without food
Lowering the dose of KISQALI can help address side effects and, in the NATALEE trial, did not impact efficacy.1,2
iDFS was similar irrespective of the relative dose intensity (RDI) of KISQALI: Patients with low (0% to <82.27%), medium (82.27% to <97.44%), and high (≥97.44%) RDI had similar iDFS outcomes (low vs high HR=0.93 [95% CI: 0.69-1.25]; medium vs high HR=0.99 [95% CI: 0.74-1.32])2
Results are based on a post hoc exploratory analysis. There was no prespecified statistical procedure controlling for type 1 error, and the results should be interpreted with caution
NATALEE was a randomized, multicenter, open-label, phase III study of KISQALI + letrozole or anastrozole (n=2549) vs letrozole or anastrozole (n=2552) for the adjuvant treatment of men and women with stage II/III HR+/HER2- eBC, including all those with node-positive or high-risk node-negative disease (eligible stages and nodal status include: anatomic stage group IIB-III, or anatomic stage group IIA that is either node positive, or node negative with histologic grade 3, or histologic grade 2 with Ki-67 ≥20% and/or high risk by gene signature testing). At a median follow-up of 33.3 months, with 509 iDFS (primary end point) events in the study (226 [8.9%] in the KISQALI arm and 283 [11.1%] in the NSAI-alone arm), iDFS at the 3-year landmark was 90.7% for KISQALI + NSAI vs 87.6% for NSAI alone (absolute difference 3.1%); there was a 25.1% relative reduction in the risk of an iDFS event; HR=0.749 (95% CI: 0.628-0.892).1,3,4
AI, aromatase inhibitor; eBC, early breast cancer; HER2-, human epidermal growth factor receptor 2-negative; HR, hazard ratio; HR+, hormone receptor-positive; iDFS, invasive disease-free survival; LHRH, luteinizing hormone-releasing hormone; NSAI, nonsteroidal aromatase inhibitor.
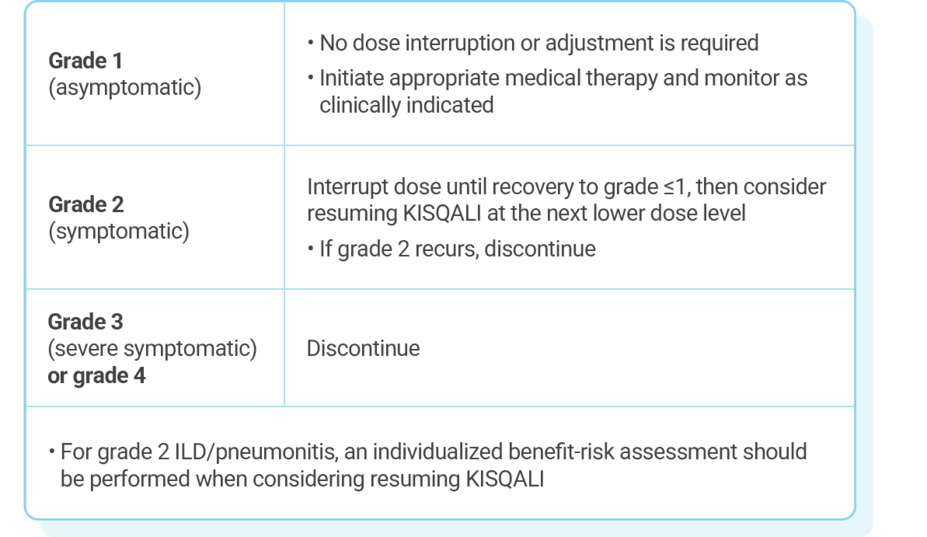
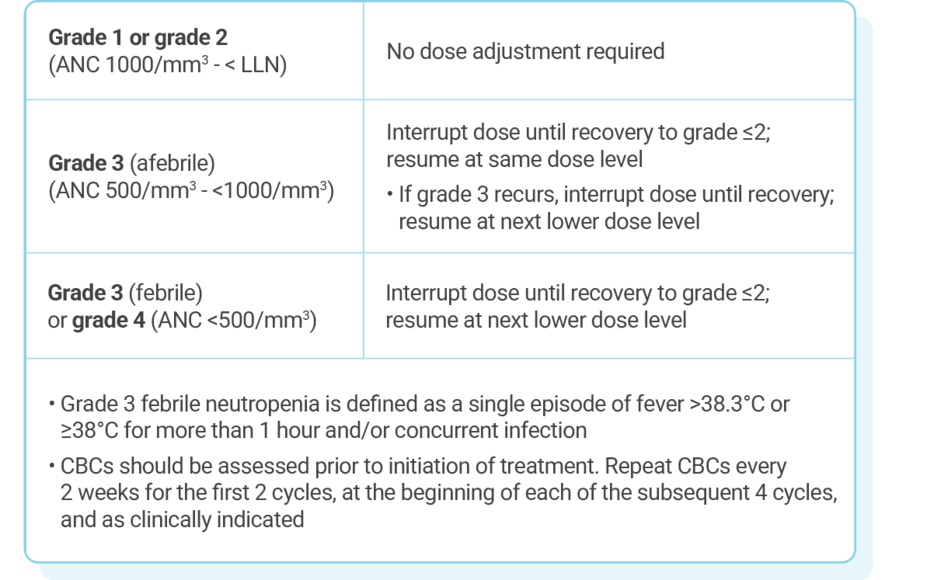
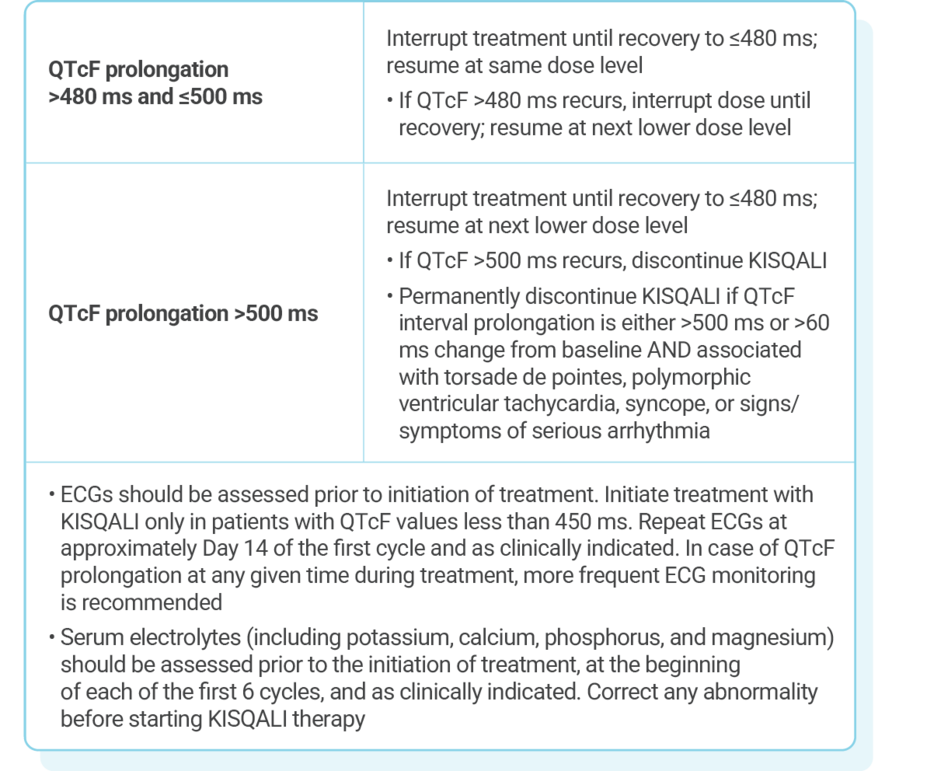
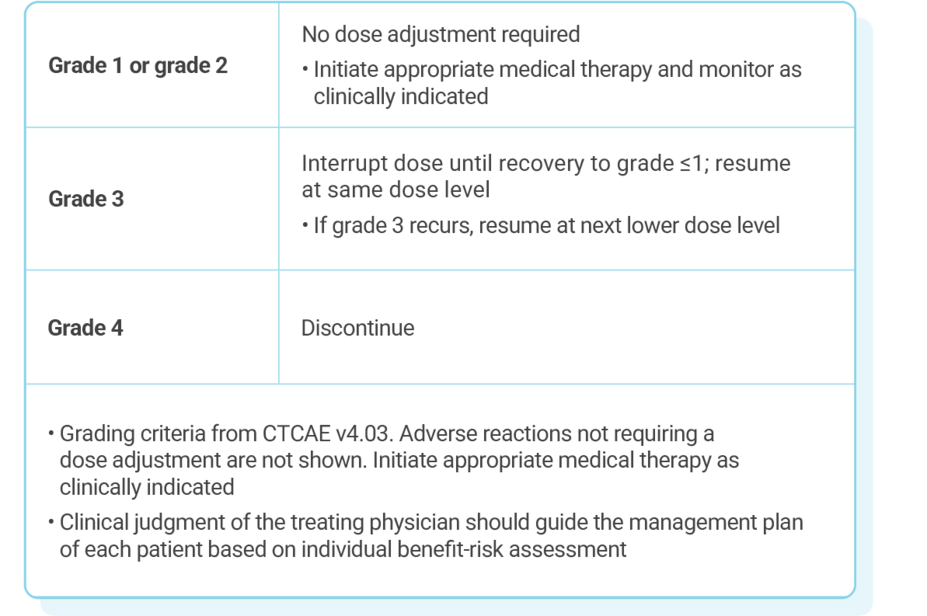
DOSE ADJUSTMENT GUIDANCE