KISQALI single-strength tablets make dose reduction simple and convenient
DOSE REDUCTIONS
Dose reductions with KISQALI mean no need for new mid-cycle prescriptions or additional costs
For patients with HR+/HER2- mBC,
KISQALI is given as 600 mg (3 x 200-mg tablets) orally, once daily (3 weeks on, 1 week off) with either AI or fulvestrant1:
AI should be taken once daily (continuously); in men and premenopausal women, an LHRH agonist should be administered according to current clinical practice guidelines; or
Fulvestrant 500 mg should be administered intramuscularly on Days 1, 15, and 29, and once monthly thereafter; in men and premenopausal women, an LHRH agonist should also be administered according to current clinical practice guidelines
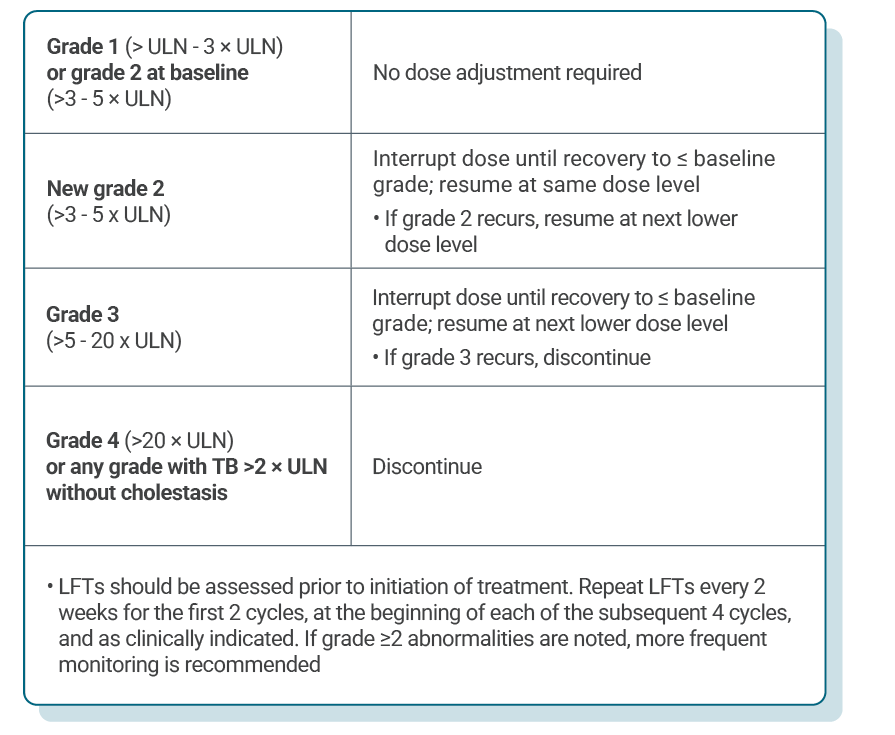
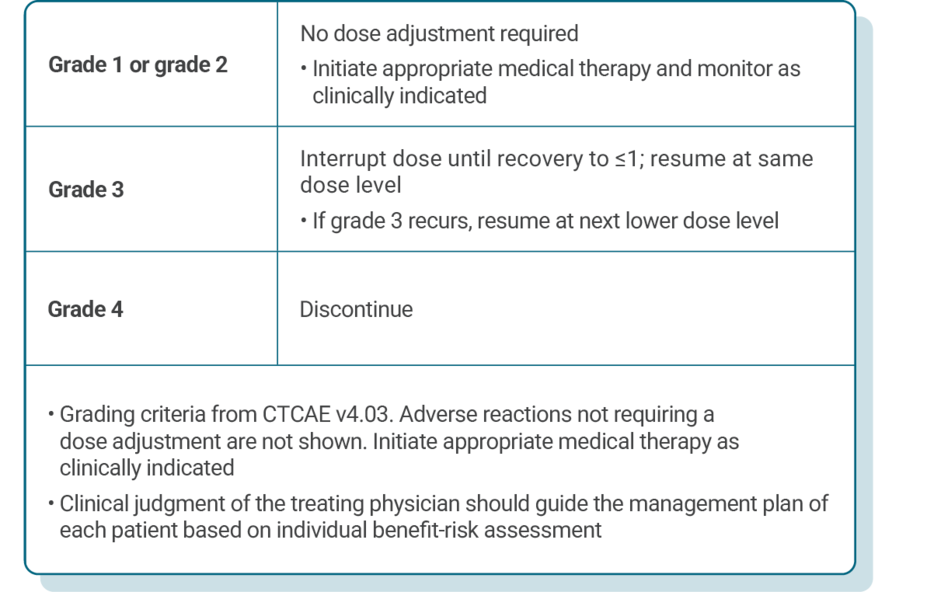
Dose adjustments for adverse reactions should be made in a stepwise order by reducing the number of tablets taken1
Dose modification of KISQALI is recommended based on individual safety and tolerability1
If dose reduction below 200 mg/day is required, discontinue treatment1
KISQALI can be taken with or without food1
AI, aromatase inhibitor; HER2-, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; LHRH, luteinizing hormone-releasing hormone; mBC, metastatic breast cancer.
EXPERT VIDEO
Expert perspective on dosing and patient adherence in HR+/HER2- mBC
“…the single-tablet strength allows for simple dose adjustments, and to me, that is game changing.” —Nick McAndrew, MD
Dr McAndrew has been compensated for his time by Novartis Pharmaceuticals Corporation.
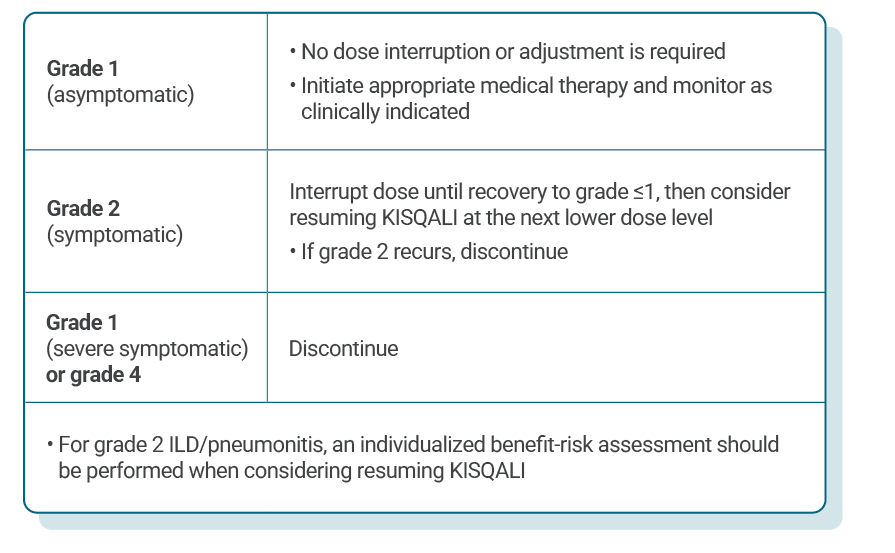
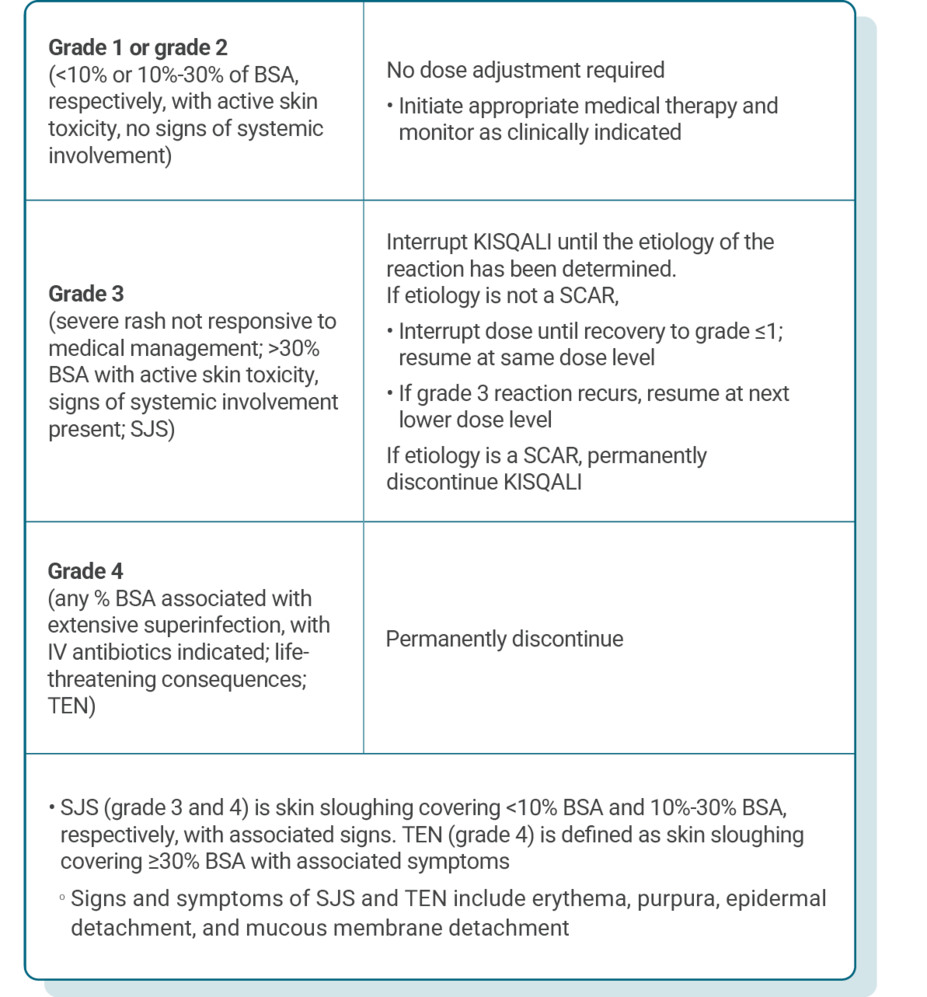
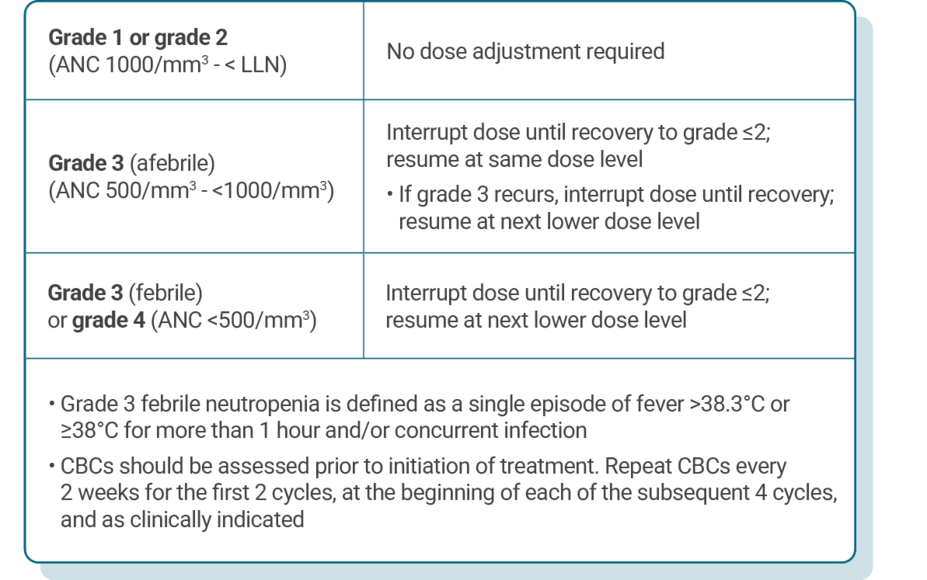
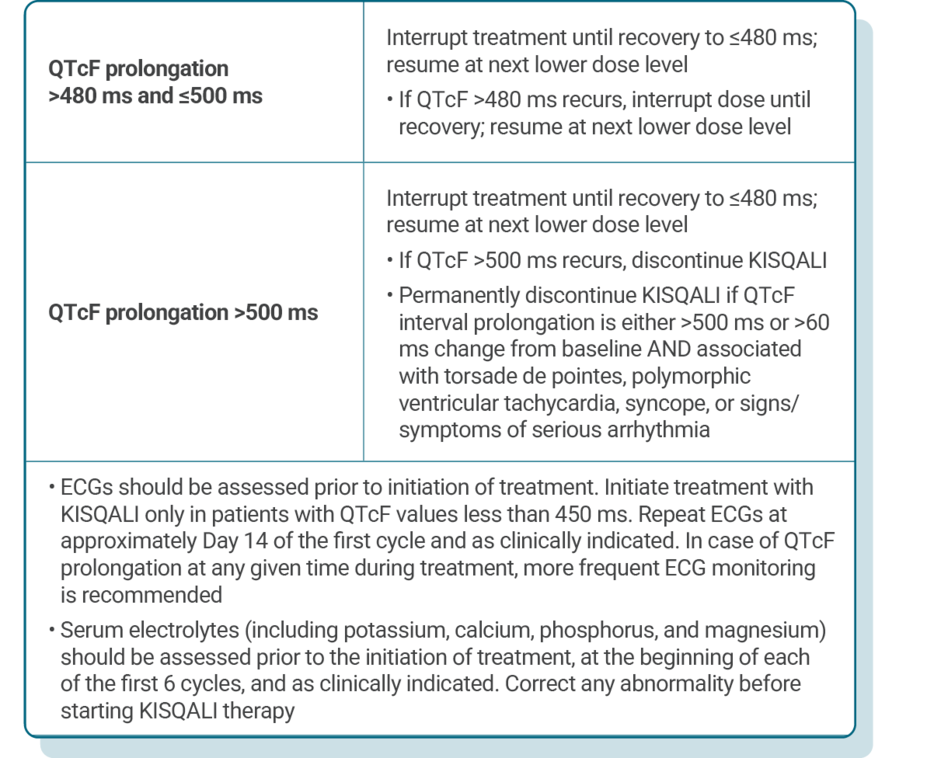
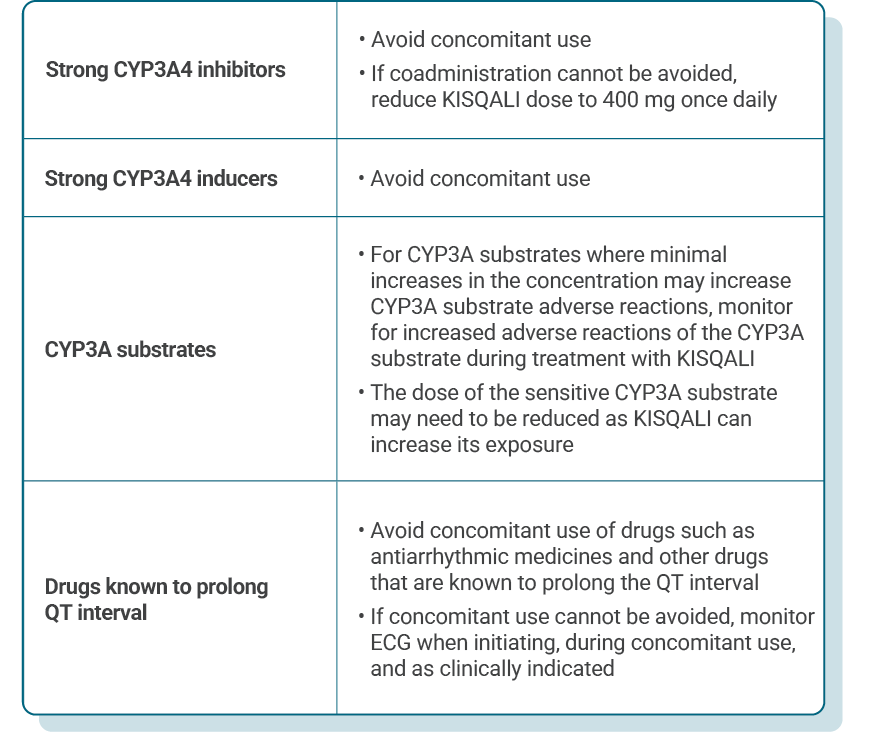
DOSE ADJUSTMENT GUIDANCE