Data across age groups, including elderly patients
OVERALL SURVIVAL
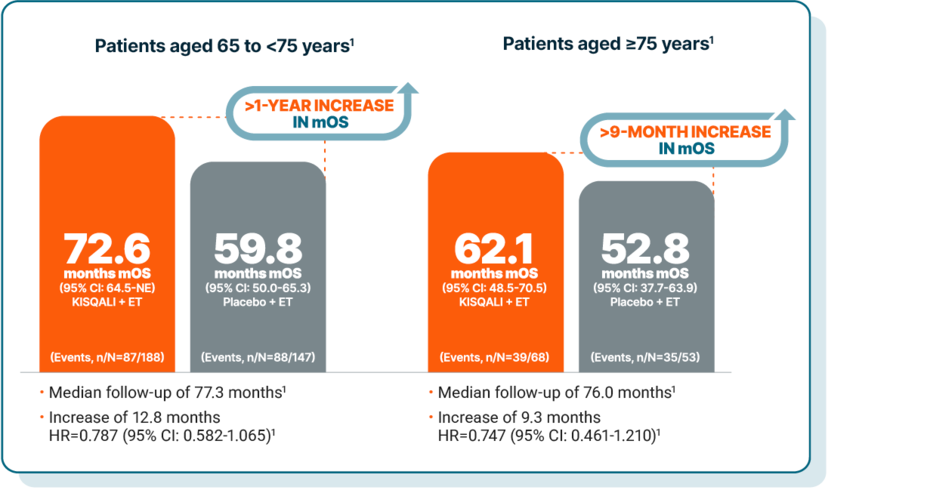
Consistent overall survival benefit across age groups, including in elderly patients
An exploratory, pooled, post hoc analysis of the MONALEESA-2, MONALEESA-3, and MONALEESA-7 studies
KISQALI prolonged mOS by 15.9 months (HR=0.686 [95% CI: 0.559-0.843]) in patients <65 years old.1
At a median follow-up of 71.2 months, mOS was1:
67.6 months with KISQALI + ET (95% CI: 59.9-NE) (Events, n/N=182/419)
51.7 months with placebo + ET (95% CI: 44.9-61.4) (Events, n/N=194/354)
Data was pooled from patients within the first-line setting in the MONALEESA-2, MONALEESA-3, and MONALEESA-7 studies. In MONALEESA-7, only the nonsteroidal aromatase inhibitor cohort was included, and patients with early relapse (≤12 months after [neo]adjuvant ET) were excluded, as their prognoses were closer to those of patients in the second-line setting.1
This pooled dataset included a total of 1229 patients across 3 different age groups; 773 (63%) were <65 years, 335 (27%) were 65 to <75 years, and 121 (10%) were ≥75 years.1
The 65 to <75 and ≥75 years age groups consisted of patients from the MONALEESA-2 and MONALEESA-3 studies. The <65 years age group consisted of patients from the MONALEESA-2, MONALEESA-3, and MONALEESA-7 studies.1
This post hoc exploratory analysis evaluated PFS, OS, time to first subsequent chemotherapy, and safety across age groups.1
The ≥75 years age group has a small sample size, and data should be interpreted with caution.
These results are exploratory and hypothesis-generating; as such, there was no statistical procedure controlling for type 1 error.
ET, endocrine therapy; HR, hazard ratio; mOS, median overall survival; NE, not estimable; OS, overall survival; PFS, progression-free survival.
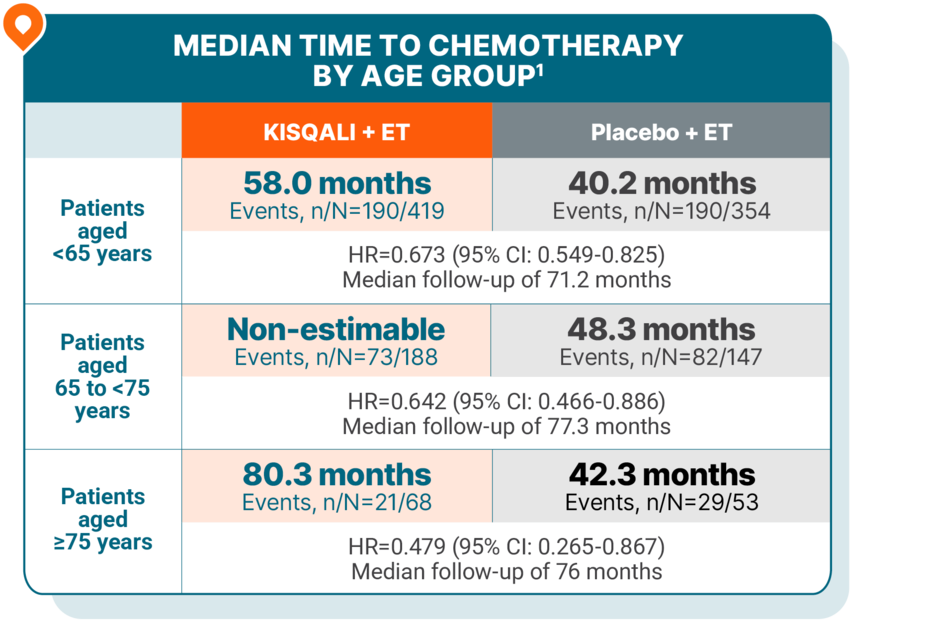
TIME TO CHEMOTHERAPY
Delayed time to chemotherapy across all age groups, including in elderly patients
Data was pooled from patients within the first-line setting in the MONALEESA-2, MONALEESA-3, and MONALEESA-7 studies. In MONALEESA-7, only the nonsteroidal aromatase inhibitor cohort was included, and patients with early relapse (≤12 months after [neo]adjuvant ET) were excluded, as their prognoses were closer to those of patients in the second-line setting.1
This pooled dataset included a total of 1229 patients across 3 different age groups; 773 (63%) were <65 years, 335 (27%) were 65 to <75 years, and 121 (10%) were ≥75 years.1
The 65 to <75 and ≥75 years age groups consisted of patients from the MONALEESA-2 and MONALEESA-3 studies. The <65 years age group consisted of patients from the MONALEESA-2, MONALEESA-3, and MONALEESA-7 studies.1
This post hoc exploratory analysis evaluated PFS, OS, time to first subsequent chemotherapy, and safety across age groups.1
The ≥75 years age group has a small sample size, and data should be interpreted with caution.
These results are exploratory and hypothesis-generating; as such, there was no statistical procedure controlling for type 1 error.
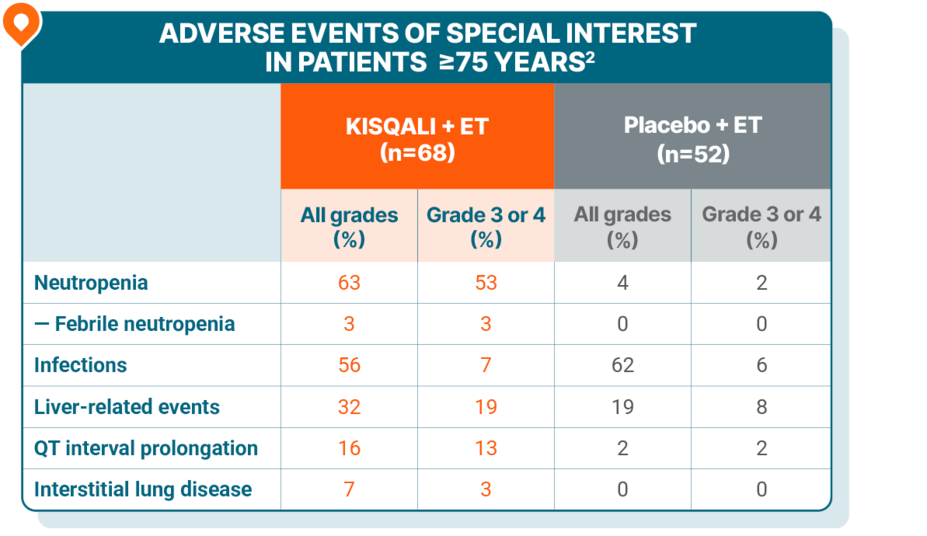
SAFETY
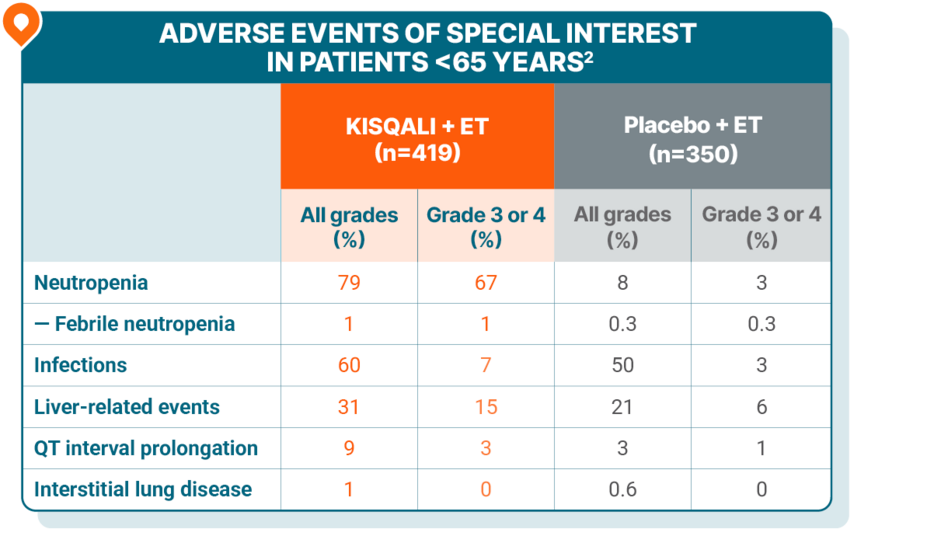
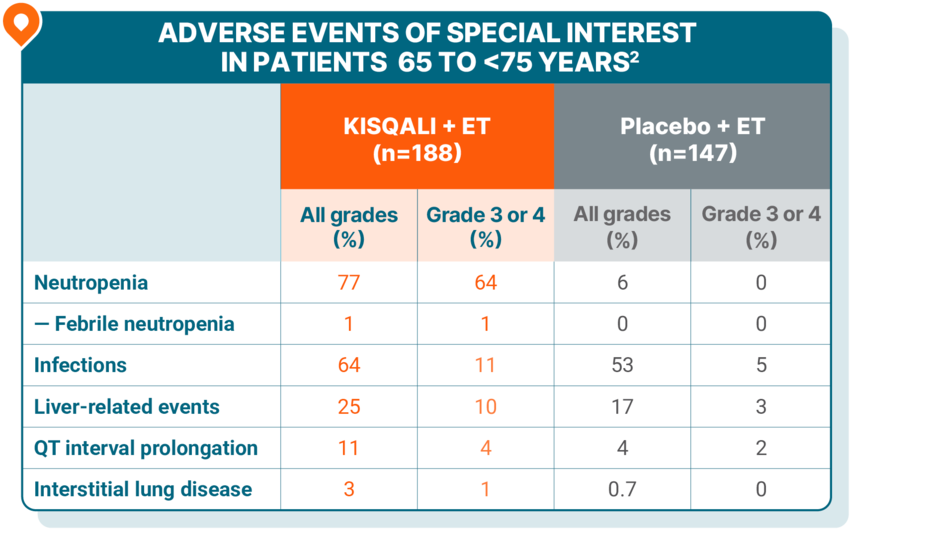
Safety was generally consistent across all age groups, including in elderly patients
Patients <65 years
No grade 5 AEs were reported.
Patients 65 to <75 years
No grade 5 AEs were reported.
Patients ≥75 years
No grade 5 AEs were reported.
Discontinuations due to AEs with KISQALI + ET1
15% in patients <65 years old
20% in patients 65 to <75 years old
41% in patients ≥75 years old
Pooled safety from pivotal MONALEESA trials (N=1065): In this pooled safety population, the most common (≥20%) adverse reactions, including laboratory abnormalities, were leukocytes decreased (95%), neutrophils decreased (93%), hemoglobin decreased (68%), lymphocytes decreased (66%), aspartate aminotransferase increased (55%), gamma-glutamyl transferase increased (53%), alanine aminotransferase increased (52%), infections (47%), nausea (47%), creatinine increased (42%), fatigue (35%), platelets decreased (34%), diarrhea (33%), vomiting (29%), headache (27%), constipation (25%), alopecia (25%), cough (24%), rash (24%), back pain (24%), and glucose serum decreased (20%). In MONALEESA-2, adverse reactions which resulted in permanent discontinuation of both KISQALI and letrozole in ≥2% of patients were alanine aminotransferase increased (5%), aspartate aminotransferase increased (3%), and vomiting (2%).3
Patients may require dose interruption, reduction, or discontinuation for ARs. Monitoring should include pulmonary symptoms, ECGs, serum electrolytes, LFTs, and CBCs. See the Warnings and Precautions section of the KISQALI Prescribing Information for risk of ILD/pneumonitis, SCARs, QT prolongation, hepatobiliary toxicity, neutropenia, and embryo-fetal toxicity.3
MONALEESA-2
Dose reductions due to ARs occurred in 45% of patients receiving KISQALI + letrozole3
Permanent discontinuations due to AEs: 7.5% with KISQALI + letrozole; 2.1% with placebo + letrozole4
MONALEESA-3
Infections included urinary and respiratory tract infections, gastroenteritis, and sepsis (1%)3
Dose reductions due to ARs occurred in 32% of patients receiving KISQALI + fulvestrant3
Permanent discontinuations due to AEs: 8.5% with KISQALI + fulvestrant; 4.1% with placebo + fulvestrant5
MONALEESA-7
Infections included urinary and respiratory tract infections, gastroenteritis, and sepsis (<1%)3
Dose reductions due to ARs occurred in 33% of patients receiving KISQALI + NSAI + goserelin3
Permanent discontinuations due to AEs in the ITT population: 4% with KISQALI + ET (NSAI or tamoxifen) + goserelin; 3% with placebo + ET (NSAI or tamoxifen) + goserelin6
KISQALI is not indicated for concomitant use with tamoxifen3
An exploratory, pooled, post hoc analysis of the MONALEESA-2, MONALEESA-3, and MONALEESA-7 studies: In 1L patients aged <65, at a median follow-up of 71.2 months, mOS was 67.6 months with KISQALI + ET (95% CI: 59.9-NE) vs 51.7 months with placebo + ET (95% CI: 44.9-61.4). In 1L patients aged 65 to 74, at a median follow-up of 77.3 months, mOS was 72.6 months with KISQALI + ET (95% CI: 64.5-NE) vs 59.8 months with placebo + ET (95% CI: 50.0-65.3). In 1L patients aged 75 or older, at a median follow-up of 76 months, mOS was 62.1 months with KISQALI + ET (95% CI: 48.5-70.5) vs 52.8 months with placebo + ET (95% CI: 37.7-63.9). The ≥75 years age group has a small sample size, and data should be interpreted with caution. These results are exploratory and hypothesis-generating; as such, there was no statistical procedure controlling for type 1 error.1
1L, first line; AE, adverse event; AR, adverse reaction; CBC, complete blood count; ECG, electrocardiogram; ILD, interstitial lung disease; ITT, intent to treat; LFT, liver function test; NSAI, nonsteroidal aromatase inhibitor; SCAR, severe cutaneous adverse reaction.