KISQALI + AI—studied in a uniquely inclusive phase III trial
NATALEE was a positive study of KISQALI efficacy and safety in the broadest range of patients at high risk of recurrence, including those with no nodal involvement.
STUDY DESIGN
NATALEE was a randomized, multicenter, open-label, phase III clinical trial of KISQALI + AI vs AI alone for the adjuvant treatment of HR+/HER2- eBC
*Men and premenopausal women also received goserelin.2
Select exclusion criteria3
Prior treatment with a CDK4/6 inhibitor
ECOG performance status ≥2
Major surgery, chemotherapy, or radiotherapy within 14 days prior to randomization
Treatment with tamoxifen, raloxifene, or AIs for reduction in risk of breast cancer and/or treatment for osteoporosis within the last 2 years
AI, aromatase inhibitor; CDK, cyclin-dependent kinase; eBC, early breast cancer; ECOG, Eastern Cooperative Oncology Group; G, grade; HER2-, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; N, nodal status; T, tumor size.
END POINTS
Reducing the risk of recurrence—including distant recurrence with incurable metastatic disease—was the primary treatment goal in NATALEE
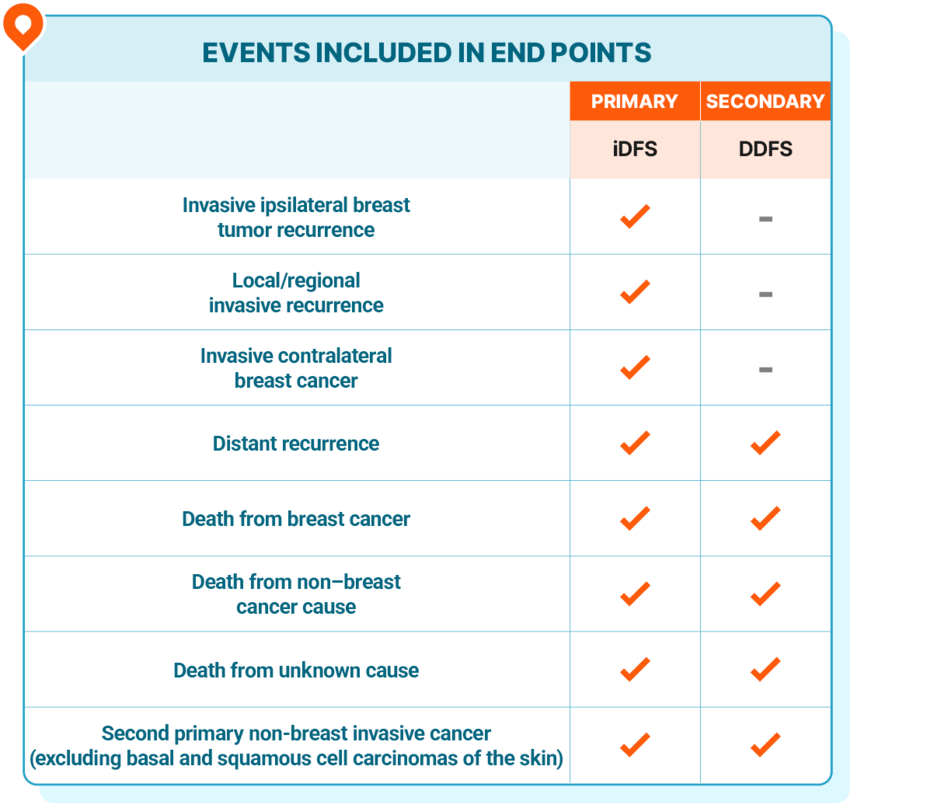
Invasive disease-free survival (iDFS), the primary end point in NATALEE, includes local and distant recurrence and death, while distant disease-free survival (DDFS) excludes local recurrence1,5
iDFS and DDFS were defined as the time from randomization to the date of the first event1,5
Overall survival (OS) is a secondary end point in NATALEE. At the time of iDFS final analysis, OS data were immature and analysis is ongoing1
Health-related quality of life (HRQOL) is an additional secondary end point in NATALEE5
PRIOR ET & DOSING
The NATALEE trial was designed to help patients start and stay on KISQALI + AI—whether new to adjuvant therapy or already on ET
Patients were eligible for KISQALI even with up to 1 year of prior ET—the most inclusive ET eligibility window of any positive CDK4/6 inhibitor trial in eBC3
NATALEE is the only positive trial of a CDK4/6 inhibitor to allow endocrine-based therapy for up to 1 year prior to randomization, so patients who began ET within the last year may still be candidates for treatment with KISQALI
Dosing was intentionally chosen for the adjuvant setting with the goal of balancing efficacy and safety3
NATALEE studied the 400-mg starting dose and 3-year duration with the goal of minimizing dose-dependent adverse reactions and adherence issues related to tolerability—with the least possible impact on efficacy
ET, endocrine therapy.
PATIENT POPULATION
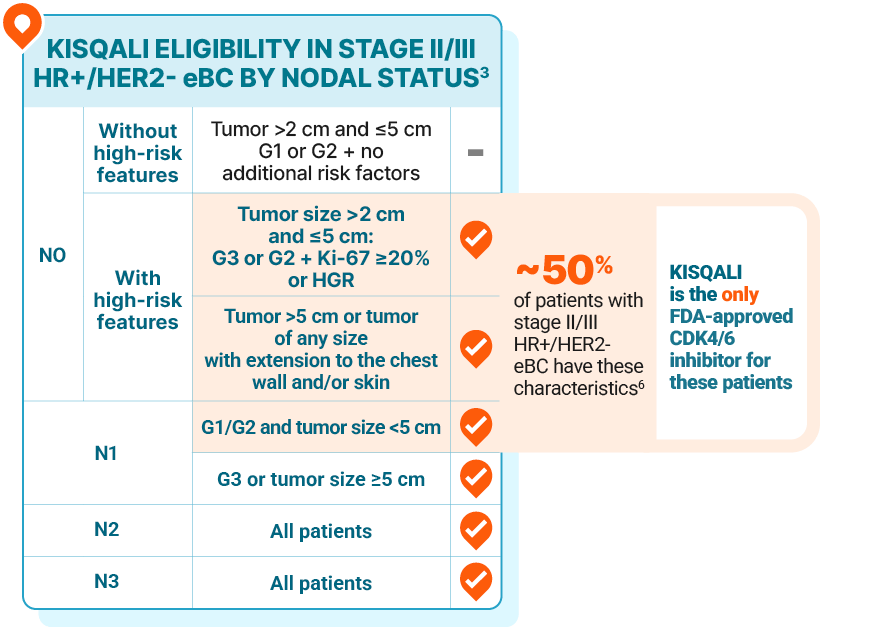
KISQALI is the only CDK4/6 inhibitor proven and FDA approved for all stage II/III node-positive and high-risk node-negative disease
High genomic risk may be determined by any of the following test scores2:
Oncotype DX®: ≥26
Prosigna® PAM50: High risk
MammaPrint®: High risk
EndoPredict®: High risk
This information is intended for reference only. Novartis does not endorse the use of any specific test or tool to help determine risk of recurrence, and there may be additional tests or tools available. The brand names above are the property of their respective trademark owners.
FDA, US Food and Drug Administration; HGR, high genomic risk.
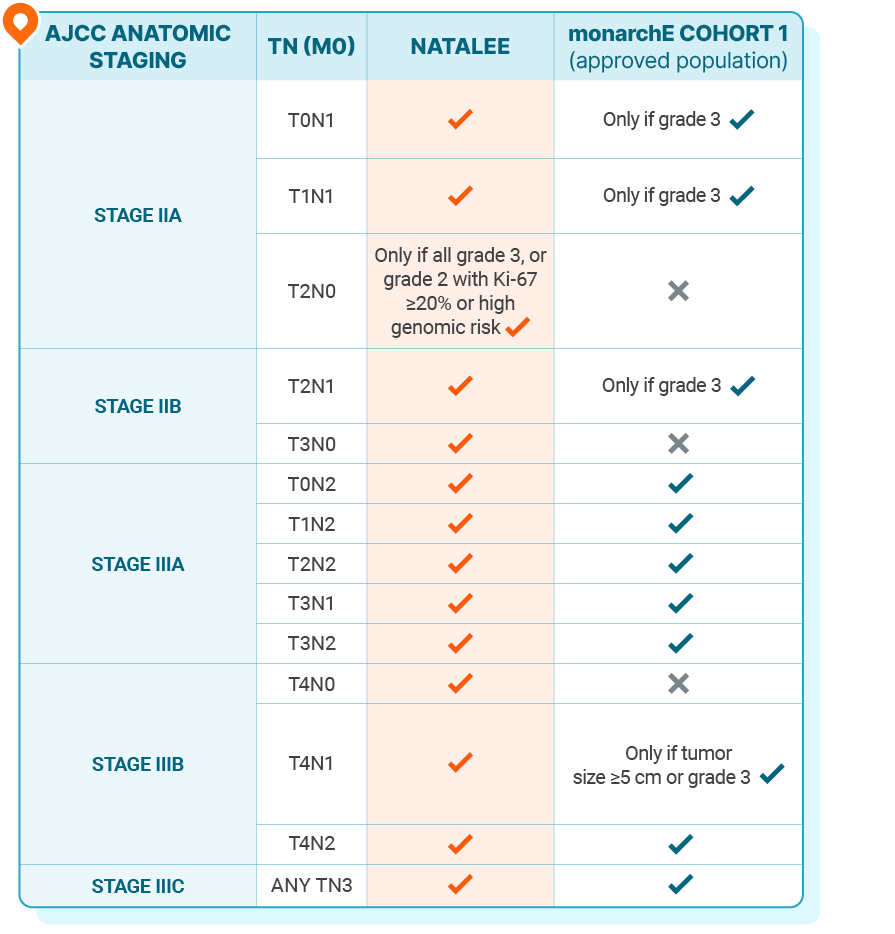
Patient populations in the NATALEE and monarchE trials3
This information is not intended to compare the efficacy or safety outcomes of the clinical trials or their treatments. No implication of superiority or inferiority is intended or should be inferred.
In NATALEE, 0.4% and 0.2% of patients had stage I disease in the KISQALI + NSAI and NSAI-alone arms, respectively. In monarchE, 0.1% of patients in the abemaciclib + ET arm had stage IA disease2,7
NATALEE allowed ET for ≤1 year prior to randomization; monarchE allowed ET for ≤3 months prior to randomization2,7
AJCC, American Joint Committee on Cancer; NSAI, nonsteroidal aromatase inhibitor.

Explore the KISQALI patient profiles
Identify eligible patients like Jasmine, Erin, and Katrice today

![Study population in NATALEE (N=5101): pre- and postmenopausal women and men with anatomic stage II or III HR+/HER2- eBC, including high-risk node-negative or node-positive disease (N0: T2 [G2 + high genomic risk or Ki-67 ≥20% or G3], T3, T4; All N1; All N2; All N3). 1:1 randomization. Study treatment was given for 3 years: KISQALI 400 mg/day (3 weeks on/1 week off) with an AI* (n=2549) vs AI alone* (n=2552); the AI was continued for 5 years. The long-term follow-up is up to ~91 months.](https://usim.beprod.kisqali-hcp.com/sites/kisqali_hcp_com/files/styles/common_width_927/public/2025-10/kisqali-early-breast-cancer-natalee-study-design-desktop_01.png?itok=wwYTF4KO)