Start your patients on KISQALI + AI with confidence
STARTING KISQALI

A few standard assessments help to ensure your patients start right away
- Complete blood count (CBC)1
- Electrocardiogram (ECG)1,*
- Electrolyte levels1,†
- Liver function tests (LFTs)1,‡

Straightforward ECG testing
- The 2 required ECGs are both completed within the first 2 weeks of treatment1
- If you are unable to perform ECGs in office, speak with your Novartis Oncology Specialist about a simple solution for fast, easy, and accurate ECG testing
AI, aromatase inhibitor; QTcF, QT interval corrected by Fridericia’s formula.
*KISQALI should only be initiated in patients with QTcF <450 ms.1
†Monitor serum electrolytes prior to the initiation of treatment, at the beginning of each of the first 6 cycles, and as clinically indicated. Correct any electrolyte abnormalities before initiating treatment.1
‡Monitor LFTs prior to the initiation of treatment, every 2 weeks for the first 2 cycles, at the beginning of each of the subsequent 4 cycles, and as clinically indicated. For LFTs, if grade ≥2 abnormalities are noted, more frequent monitoring is recommended.1
Additional monitoring may be required as clinically indicated.
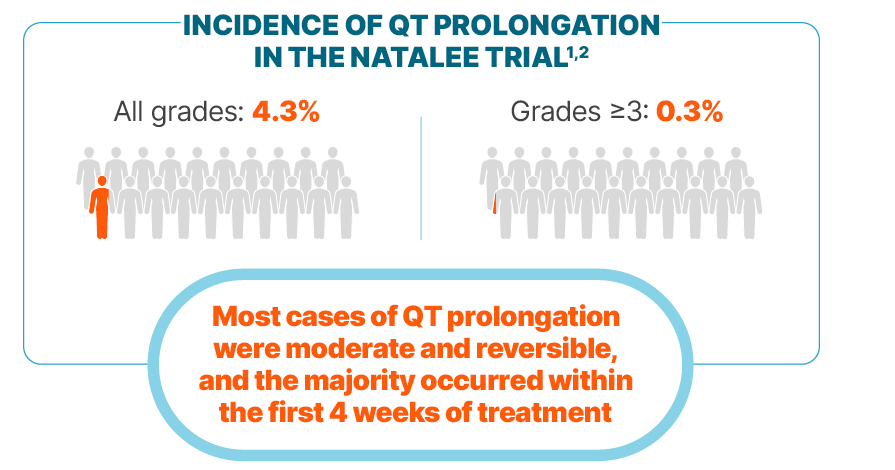
Incidence of QT prolongation observed with KISQALI was low
Among cases of QT prolongation1:
0.3% had a >500 ms postbaseline QTcF value
2% had a >60 ms increase from baseline in QTcF interval
There were no reported cases of torsades de pointes
DOSING & ADJUSTMENTS
For your patients with stage II/III HR+/HER2- eBC at high risk of recurrence, in combination with an AI,
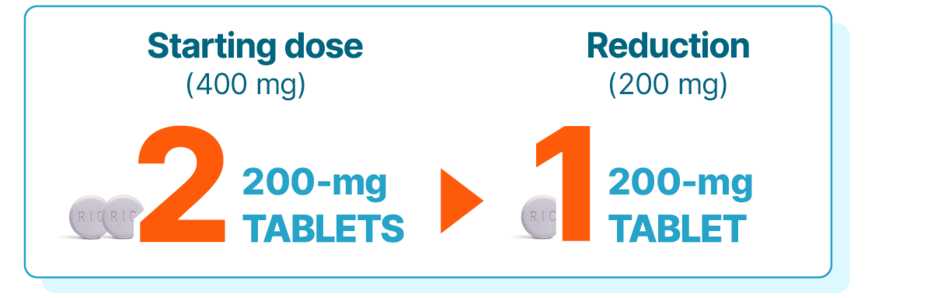
Start with KISQALI 400 mg—the starting dose chosen to help reduce both the risk of recurrence and dose-dependent ARs
KISQALI single-strength tablets make dose reduction simple and convenient—with no need for new mid-cycle prescriptions or additional costs1
KISQALI is given as 400 mg (2 x 200-mg tablets) orally, once daily (3 weeks on, 1 week off) for 36 months with an AI1
Review the full Prescribing Information for recommended dosing of selected AI
An LHRH agonist should be used concomitantly with AI in men and premenopausal women
Patients should continue treatment for 3 years or until disease recurrence or unacceptable toxicity
Dose adjustments for adverse reactions should be made by reducing the number of tablets taken. Dose modification of KISQALI is recommended based on individual safety and tolerability. If dose reduction below 200 mg/day is required, discontinue treatment.
Lowering the dose of KISQALI can help address side effects and, in the NATALEE trial, did not impact efficacy.1,3
iDFS was similar irrespective of the relative dose intensity (RDI) of KISQALI: Patients with low (0% to <82.27%), medium (82.27% to <97.44%), and high (≥97.44%) RDI had similar iDFS outcomes (low vs high HR=0.93 [95% CI: 0.69-1.25]; medium vs high HR=0.99 [95% CI: 0.74-1.32])3
Results are based on a post hoc exploratory analysis. There was no prespecified statistical procedure controlling for type 1 error, and the results should be interpreted with caution
NATALEE was a randomized, multicenter, open-label, phase III study of KISQALI + letrozole or anastrozole (n=2549) vs letrozole or anastrozole (n=2552) for the adjuvant treatment of men and women with stage II/III HR+/HER2- eBC, including all those with node-positive or high-risk node-negative disease (eligible stages and nodal status include: anatomic stage group IIB-III, or anatomic stage group IIA that is either node positive, or node negative with histologic grade 3, or histologic grade 2 with Ki-67 ≥20% and/or high risk by gene signature testing). At a median follow-up of 33.3 months, with 509 iDFS (primary end point) events in the study (226 [8.9%] in the KISQALI arm and 283 [11.1%] in the NSAI-alone arm), iDFS at the 3-year landmark was 90.7% for KISQALI + NSAI vs 87.6% for NSAI alone (absolute difference 3.1%); there was a 25.1% relative reduction in the risk of an iDFS event; HR=0.749 (95% CI: 0.628-0.892).1,4
AR, adverse reaction; eBC, early breast cancer; HER2-, human epidermal growth factor receptor 2-negative; HR, hazard ratio; HR+, hormone receptor-positive; iDFS, invasive disease-free survival; LHRH, luteinizing hormone-releasing hormone; NSAI, nonsteroidal aromatase inhibitor.
NOVARTIS PATIENT SUPPORT™
A dedicated team for your patients
Novartis Patient Support is a comprehensive program that is designed to help your eligible patients start, stay, and save on KISQALI.
We support your patient’s journey with:
Dedicated assistance with access and reimbursement
Personalized support for your patients on therapy
Single points of contact for you and your patients
Get your patients started by downloading the Start Form.
Unrestricted coverage from MMIT data as of July 2025.5
Novartis does not guarantee payment or coverage for any product or service. Actual coverage and reimbursement decisions are made by individual payers following receipt of claims. Coverage is subject to change by the relevant payer.