MEET KATRICE: She was recently diagnosed with stage III (T2N2) HR+/HER2- eBC
Find out why KISQALI + AI is right for patients with stage II/III HR+/HER2- eBC at high risk of recurrence.
BACKGROUND
Get to know Katrice

Katrice is 63 years old and lives a well-rounded life as a daughter, wife, and colleague. She's busy with a DIY home renovation project but stays grounded through a consistent yoga practice and quality time with friends.
- Katrice found a small lump in one of her breasts. She immediately called her primary care doctor, who ordered a mammogram
- A biopsy confirmed she had HR+/HER2- breast cancer that had spread to her lymph nodes
- She had a mastectomy and rang the bell after completing chemotherapy; she is now on hormone therapy
Discover more: download Katrice's patient profile.
EVALUATION
Katrice’s clinical evaluation
Age | 63 |
Menopausal status | Postmenopausal |
Clinical features |
|
Hormone receptor assay status | ER+/PR+/HER2- |
ECOG PS | 1 |
Prior therapy | Mastectomy; adjuvant chemotherapy |
Current therapy | Hormone therapy |
DIAGNOSIS: Stage III (T2N2) HR+/HER2- eBC
RISK OF RECURRENCE
Estimated risk of recurrence for patients with stage III HR+/HER2 - eBC
3-year risk of recurrence is based on iDFS outcomes among patients with HR+/HER2- eBC who received ET in select CDK4/6 inhibitor clinical trials. Data are from control arms only; no comparisons should be made between results from CDK4/6 inhibitor trial arms.1,2
CDK, cyclin-dependent kinase; ET, endocrine therapy; iDFS, invasive disease-free survival.
ANALYSIS
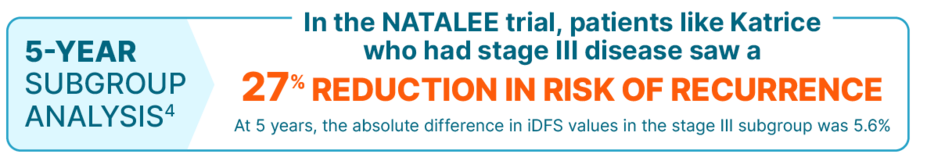
KISQALI + AI consistently reduced the risk of recurrence in the broadest range of patients with stage II/III HR+/HER2- eBC at high risk of recurrence
The iDFS benefit seen in the stage III subgroup was consistent with the overall population
In the 5-year prespecified analysis, at a median follow-up of 55.4 months, reduction in risk of recurrence (iDFS) at the 5-year landmark was 85.5% for KISQALI + AI vs 81.0% for AI alone (absolute difference 4.5%); there was a 28.4% relative reduction in the risk of an iDFS event; HR=0.716 (95% CI: 0.618-0.829).4
The 5-year analysis was prespecified and observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.
iDFS was defined as the time from randomization to the date of the first event of local invasive breast cancer recurrence, regional invasive recurrence, distant recurrence, contralateral invasive breast cancer, second primary non-breast invasive cancer (excluding basal and squamous cell carcinomas of the skin), or death (any cause).3
NATALEE was a randomized, multicenter, open-label, phase III study of KISQALI + letrozole or anastrozole (n=2549) vs letrozole or anastrozole (n=2552) for the adjuvant treatment of men and women with stage II/III HR+/HER2- eBC, including all those with node-positive or high-risk node-negative disease (eligible stages and nodal status include: anatomic stage group IIB-III, or anatomic stage group IIA that is either node positive, or node negative with histologic grade 3, or histologic grade 2 with Ki-67 ≥20% and/or high risk by gene signature testing). At a median follow-up of 33.3 months, with 509 iDFS (primary end point) events in the study (226 [8.9%] in the KISQALI arm and 283 [11.1%] in the NSAI-alone arm), iDFS at the 3-year landmark was 90.7% for KISQALI + NSAI vs 87.6% for NSAI alone (absolute difference 3.1%); there was a 25.1% relative reduction in the risk of an iDFS event; HR=0.749 (95% CI: 0.628-0.892). At a median follow-up of 50.0 months, iDFS for the prespecified stage III subgroup at the 5-year landmark was 81.2% for KISQALI + NSAI vs 75.6% for NSAI alone; HR=0.730 (95% CI: 0.615-0.865). In the 5-year prespecified analysis, prespecified subgroups included anatomic stage (stage II: HR=0.660 [95% CI: 0.493-0.884]; stage III: HR=0.730 [95% CI: 0.615-0.865]), nodal status (N0: HR=0.606 [95% CI: 0.372-0.986]; N1, N2, N3: HR=0.737 [95% CI: 0.631-0.860]), and menopausal status (premenopausal/men: HR=0.714 [95% CI: 0.565-0.902]; postmenopausal: HR=0.734 [95% CI: 0.608-0.887]). The 5-year analysis was prespecified and observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.3-7
No new safety signals were observed with KISQALI in the adjuvant setting
View safety from the NATALEE trial.
KISQALI + AI consistently improved iDFS across subgroups, regardless of stage, nodal status, or menopausal status
KISQALI may be right for a variety of patients with stage II/III HR+/HER2- eBC.
KISQALI is approved for patients with stage II/III high-risk node-negative or node-positive HR+/HER2- eBC
Regardless of nodal status, tumor size, tumor grade, age (≥18 years), or menopausal status—consider KISQALI for your patients.
Patients with stage IIA, T2N0 HR+/HER2- eBC must meet the following criteria to be eligible for treatment with KISQALI: grade 3, or grade 2 with Ki-67 ≥20% or high genomic risk.7