NATALEE safety profile
The NATALEE trial was designed to maximize the efficacy benefit of KISQALI while minimizing dose-dependent ARs and adherence issues related to tolerability.
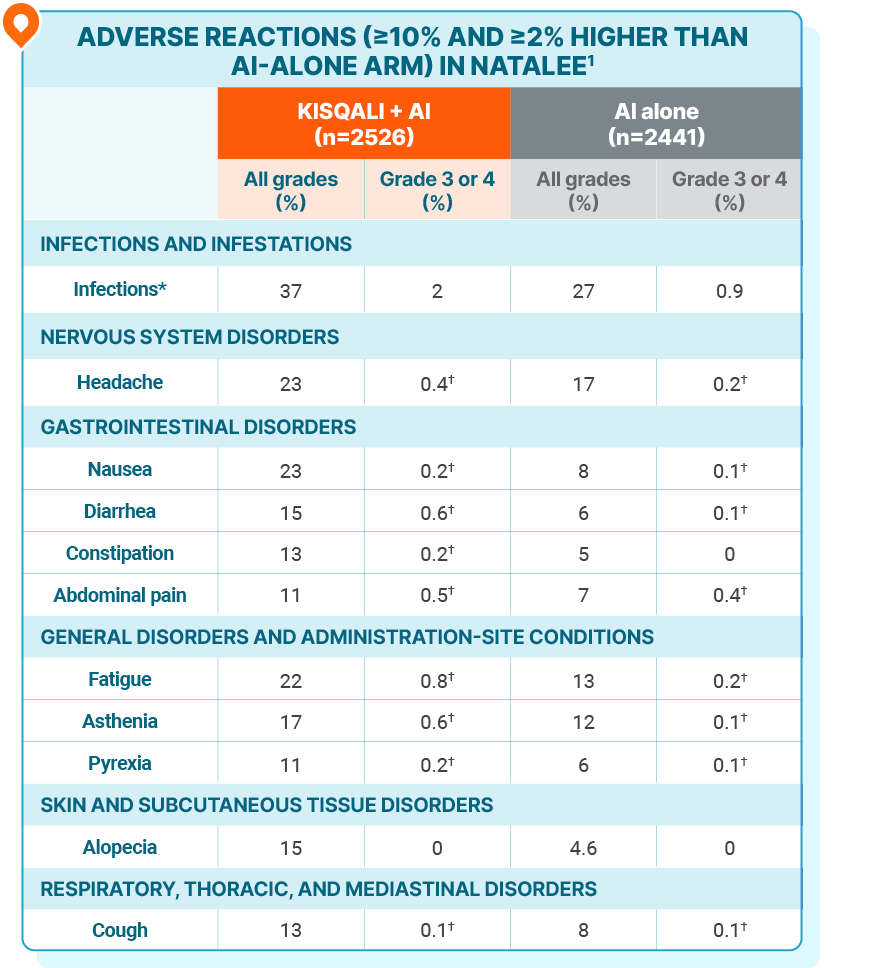
ADVERSE REACTIONS
No new safety signals were observed with KISQALI + AI in the adjuvant setting
With 33.3 months of follow-up
Grading according to CTCAE version 4.03.
*Infections included urinary and respiratory tract infections.
†Only includes grade 3 ARs.
The most common ARs (occurring in ≥20% of patients treated with KISQALI), including laboratory abnormalities, were decrease in lymphocytes, decrease in leukocytes, decrease in neutrophils, decrease in hemoglobin, increase in ALT, increase in AST, infections, increase in creatinine, decrease in platelets, headache, nausea, and fatigue1
The most common grade ≥3 ARs, including laboratory abnormalities, occurring in ≥5% of patients were decrease in neutrophils, decrease in leukocytes, decrease in lymphocytes, increase in ALT, and increase in AST1
Fatal ARs occurred in 0.6% of patients who received KISQALI. Fatal ARs in ≥0.1% of patients receiving KISQALI included COVID-19 or COVID-19 pneumonia (0.2%) and pulmonary embolism (0.1%)1
In the NATALEE trial, no new safety signals were observed at 5 years of follow-up2
AI, aromatase inhibitor; ALT, alanine aminotransferase; AR, adverse reaction; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events.
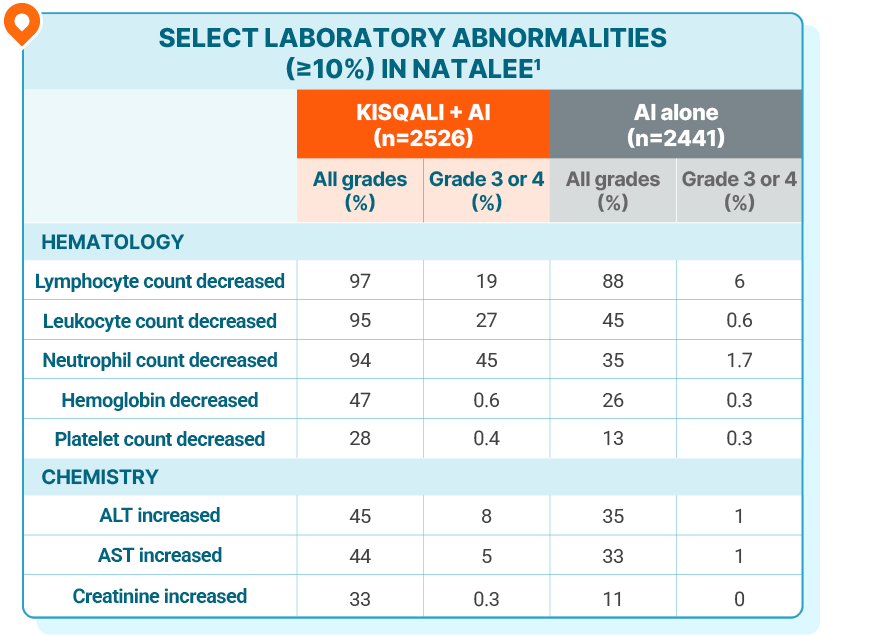
LAB ABNORMALITIES
No new lab abnormalities were observed with KISQALI in the adjuvant setting
With 33.3 months of follow-up
Grade 4 increases in ALT (1.5%) and AST (0.8%) were reported in the KISQALI + AI arm1
Drug-induced liver injury was reported in 9 patients (0.4%), of which 5 were grade ≥3, and 8 had resolved as of the data cutoff. There were 8 (0.3%) clinically confirmed Hy’s Law cases (including 4 out of 9 drug-induced liver injury mentioned above), 6 of which had resolved within 303 days and 2 of which were improving, all after discontinuation of KISQALI1
In the NATALEE trial, no new safety signals were observed at 5 years of follow-up2
DOSE REDUCTION & DISCONTINUATION
With KISQALI, most adverse reactions were manageable and reversible with dose reduction, which may have helped patients remain on therapy
In NATALEE, the leading causes of KISQALI + AI discontinuation (occurring in ≥2% of patients) were increases in ALT or AST (8%).1
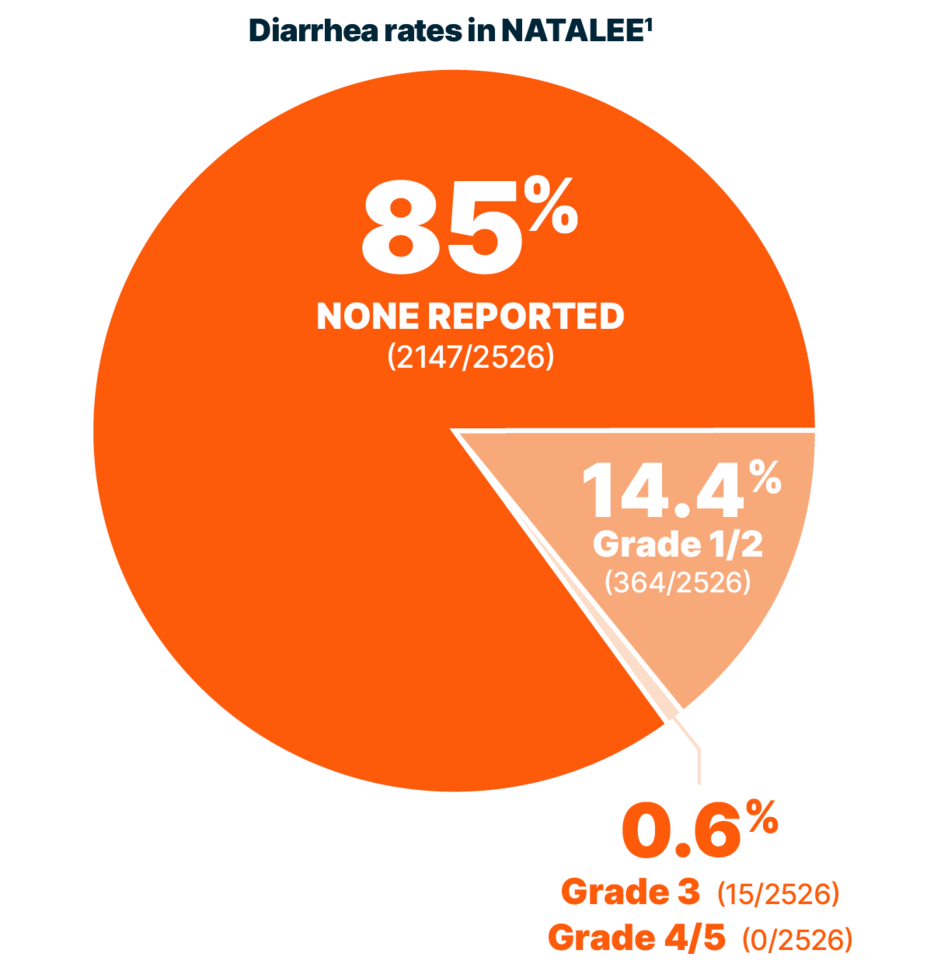
DIARRHEA RATES
Reported rates of diarrhea were low with KISQALI
Diarrhea can be disruptive in many ways—from a daily, unpredictable inconvenience to a debilitating, even life-threatening condition5
Grade 1: <4 stools/day over baseline; mild increase in ostomy output
Grade 2: 4 to 6 stools per day over baseline; moderate increase in ostomy output; limiting instrumental ADL
Grade 3: ≥7 stools per day over baseline; hospitalization indicated; severe increase in ostomy output; limiting self-care ADL
Grade 4: Life-threatening; urgent intervention indicated
Grade 5: Death
ADL, activities of daily living.
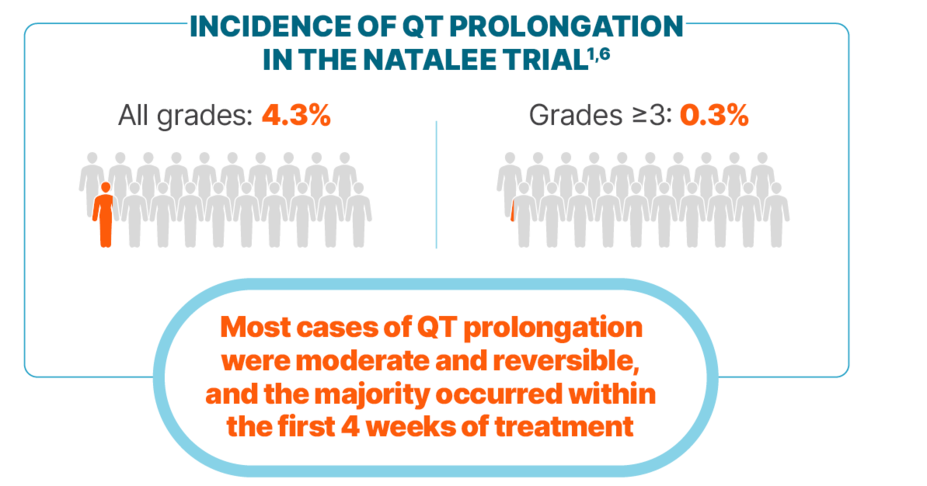
QT PROLONGATION
Incidence of QT prolongation observed with KISQALI was low
Among cases of QT prolongation1:
0.3% had a >500 ms postbaseline QTcF value
2% had a >60 ms increase from baseline in QTcF interval
There were no reported cases of torsades de pointes
QTcF, QT interval corrected by Fridericia’s formula.
Scheduled assessments help to ensure your patients start KISQALI with confidence
Review the assessments schedule
The majority of adverse reactions with KISQALI were manageable and reversible
Review the dose adjustment guidance for patients with stage II/III HR+/HER2- eBC at high risk of recurrence