With KISQALI, treatment is about more life for living
KISQALI + AI or fulvestrant is proven to help a broad range of patients with HR+/HER2- mBC live longer—and that means more time doing what they love.
NCCN GUIDELINES®
NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
AI, aromatase inhibitor; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; HER2-, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; mBC, metastatic breast cancer.
OS RESULTS
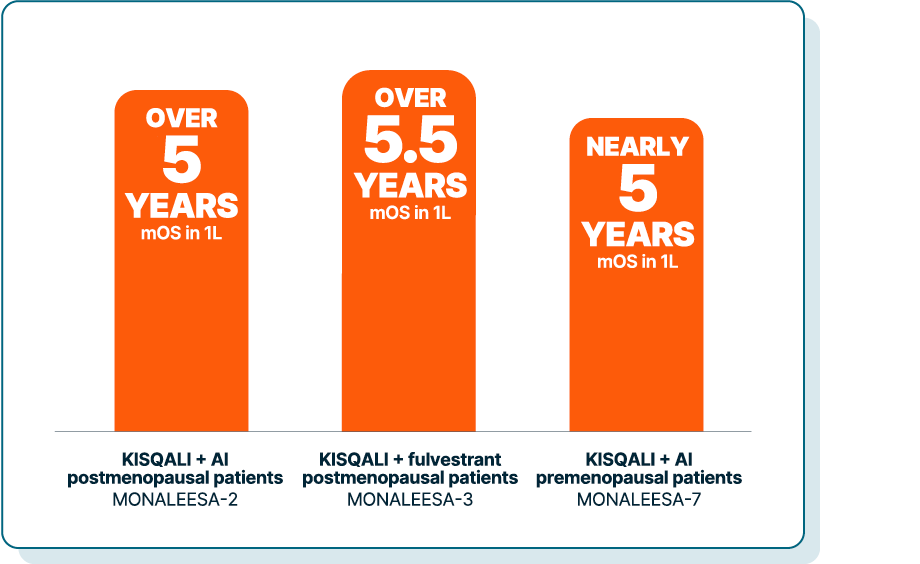
KISQALI—the only CDK4/6 inhibitor to achieve statistically significant OS in a broad range of patients across 3 phase III trials2,3
1L refers to patients with mBC across all trials.
MONALEESA study information
MONALEESA-2 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + letrozole (n=334) vs placebo + letrozole (n=334) in postmenopausal patients with HR+/HER2- mBC who received no prior therapy for advanced disease. OS was a secondary end point; PFS was the primary end point. At a median follow-up of 80 months, mOS was 63.9 months with KISQALI + letrozole (95% CI: 52.4-71.0) vs 51.4 months with placebo + letrozole (95% CI: 47.2-59.7); HR=0.765 (95% CI: 0.628-0.932); P=0.004.2,4,5
MONALEESA-3 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + fulvestrant (n=484) vs placebo + fulvestrant (n=242) in postmenopausal patients with HR+/HER2- mBC who received no or only 1 line of prior ET for advanced disease. OS was a secondary end point; PFS was the primary end point. In an exploratory analysis of a 1L subgroup of patients receiving KISQALI + fulvestrant (n=237) or placebo + fulvestrant (n=128), at a median follow-up of 71 months mOS was 67.6 months with KISQALI + fulvestrant (95% CI: 59.6-NR) vs 51.8 months with placebo + fulvestrant (95% CI: 40.4-61.2); HR=0.673 (95% CI: 0.504-0.899). At a median follow-up of 39 months, statistical significance was established for overall survival in the ITT population; HR=0.724 (95% CI: 0.568-0.924); P=0.00455. Results from the 71-month analysis were not prespecified and were observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.2,6-8
MONALEESA-7 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + ET (NSAI or tamoxifen) + goserelin (n=335) vs placebo + ET (NSAI or tamoxifen) + goserelin (n=337) (ITT) in premenopausal patients with HR+/HER2- mBC who received no prior ET for advanced disease. KISQALI is not indicated for concomitant use with tamoxifen. Efficacy results are from a prespecified subgroup analysis of 495 patients who received KISQALI (n=248) or placebo (n=247) with an NSAI + goserelin and were not powered to show statistical significance. OS was a secondary end point; PFS was the primary end point. At a median follow-up of 54 months (exploratory analysis), mOS was 58.7 months with KISQALI + NSAI + goserelin (95% CI: 48.5-NR) vs 47.7 months with placebo + NSAI + goserelin (95% CI: 41.2-55.4); HR=0.798 (95% CI: 0.615-1.035). At a median follow-up of 35 months, statistical significance was established for overall survival in the ITT population; HR=0.71 (95% CI: 0.54-0.95); P=0.00973. Results from the 54-month analysis were not prespecified and were observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.2,3,9-11
Take a closer look at the MONALEESA trials
Swipe to explore the MONALEESA trials →
Aggressive disease & visceral metastases
Explore outcomes from the RIGHT Choice trial and a pooled MONALEESA analysis for KISQALI + ET in patients with HR+/HER2- mBC who had aggressive disease and visceral metastases without visceral crisis